name reaction of aldehyde ketone and carboxylic acid 14.9 aldehydes and ketones: structure and names
Hey, there! Today we are going to talk about a very important topic in organic chemistry - Aldehydes and Ketones. So, let’s dive in! Aldehydes and ketones are two types of organic compounds that contain the carbonyl group, C=O. They are widely used in many industrial processes and are also found in nature. The basic difference between aldehydes and ketones is that aldehydes have a carbonyl group at the end of the carbon chain, whereas ketones have a carbonyl group in the middle of the carbon chain. Let’s start with aldehydes. The most common aldehyde is formaldehyde, which is used as a preservative in biological samples. It has a pungent smell and is toxic in nature. Other important aldehydes include acetaldehyde, which is responsible for the hangover after drinking alcohol, and benzaldehyde, which is commonly used as a flavoring agent in foods and perfumes. Now, let’s talk about ketones. The most common ketone is acetone, which is used as a solvent and is also found in nail polish removers. Other important ketones include propanone, which is commonly used in the production of plastics, and methyl ethyl ketone, which is used as a solvent in the production of paints and varnishes. The structural formula of aldehydes and ketones is very similar. The carbonyl group consists of a carbon atom doubly bonded to an oxygen atom. In aldehydes, the carbonyl group is at the end of the carbon chain and is attached to a hydrogen atom and an R group. In ketones, the carbonyl group is in the middle of the carbon chain and is attached to two R groups. Now, let’s take a look at the images we have here. The first one shows the structure and names of aldehydes and ketones. As you can see, the carbonyl group is highlighted in red and is attached to different groups in aldehydes and ketones. The image also provides the names of some important aldehydes and ketones along with their molecular formula. The second image shows the functional groups present in aldehydes, ketones, carboxylic acids, and esters. As you can see, the carbonyl group is present in all four types of compounds. The image also provides examples of each type of compound and their structural formula. In conclusion, aldehydes and ketones are important organic compounds that have a wide range of industrial and biological applications. They are similar in structure, but differ in the position of the carbonyl group in the carbon chain. The images provided here provide an understanding of the structure and functional groups present in aldehydes and ketones. I hope this post was informative and helpful to you!
If you are looking for 14.9 Aldehydes and Ketones: Structure and Names | The Basics of General you’ve visit to the right web. We have 5 Pics about 14.9 Aldehydes and Ketones: Structure and Names | The Basics of General like 20.3: Aldehydes, Ketones, Carboxylic Acids, and Esters - Chemistry, 14.9 Aldehydes and Ketones: Structure and Names | The Basics of General and also 20.3: Aldehydes, Ketones, Carboxylic Acids, and Esters - Chemistry. Read more:
14.9 Aldehydes And Ketones: Structure And Names | The Basics Of General
 courses.lumenlearning.comaldehydes organic general structure ketones chemistry biological names naming oxidation four first acid carboxylic group common basics acids carbonyl compounds
courses.lumenlearning.comaldehydes organic general structure ketones chemistry biological names naming oxidation four first acid carboxylic group common basics acids carbonyl compounds
20.3: Aldehydes, Ketones, Carboxylic Acids, And Esters - Chemistry
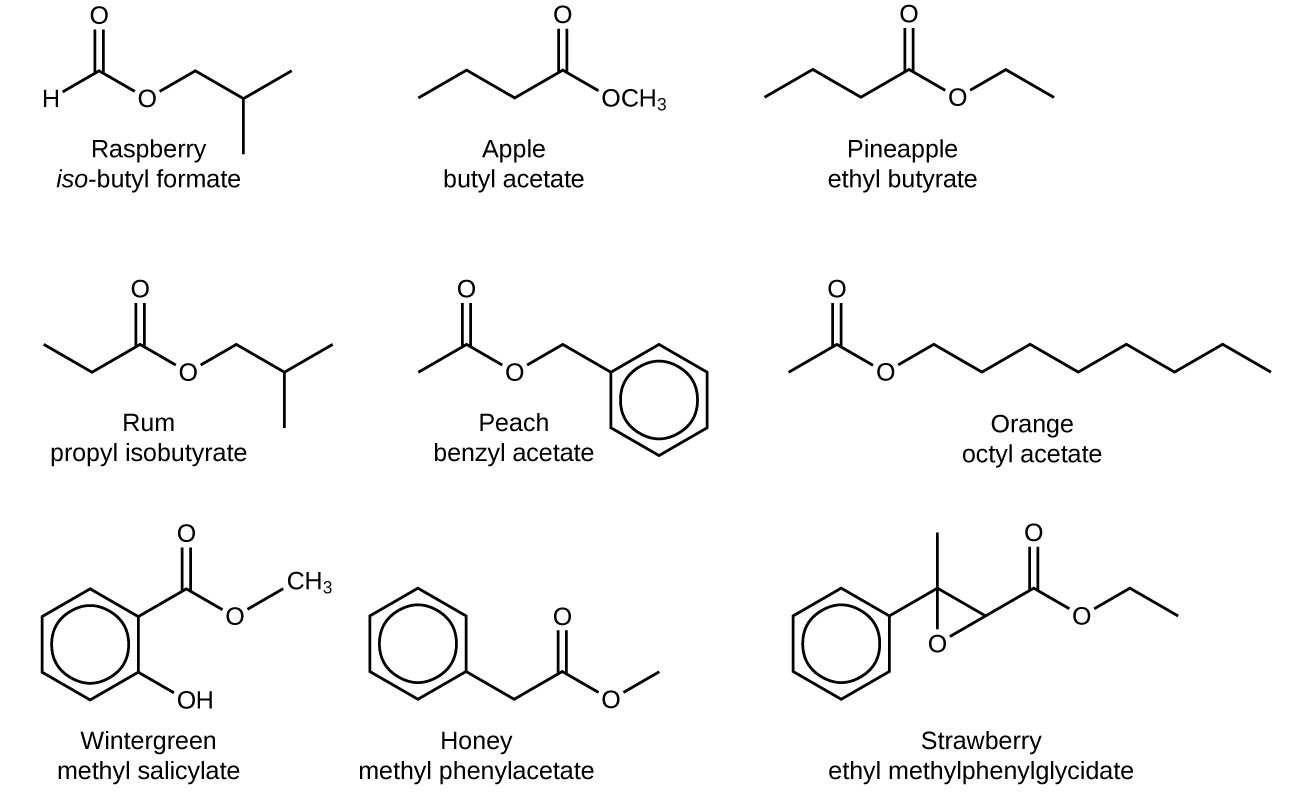 chem.libretexts.orgcarboxylic esters acids aldehydes ketones chemistry which line bond structures organic group double carbonyl their ethyl atom odors contain butyl
chem.libretexts.orgcarboxylic esters acids aldehydes ketones chemistry which line bond structures organic group double carbonyl their ethyl atom odors contain butyl
Aldehydes, Ketones And Carboxylic Acids
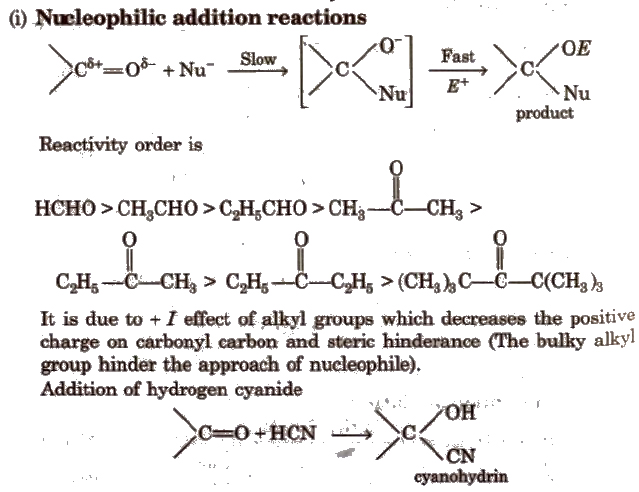 www.aayushwagle.com.npcarboxylic aldehydes ketones acids reaction reactions addition chemical
www.aayushwagle.com.npcarboxylic aldehydes ketones acids reaction reactions addition chemical
Pin On Organic Chemistry
 in.pinterest.comaldehyde ketone chemistry carboxylic reaction acid cheat sheet reactions organic level class notes teaching help mechanisms choose board science school
in.pinterest.comaldehyde ketone chemistry carboxylic reaction acid cheat sheet reactions organic level class notes teaching help mechanisms choose board science school
Aldehydes & Ketones | Virtual Textbook Of Organic Chemistry
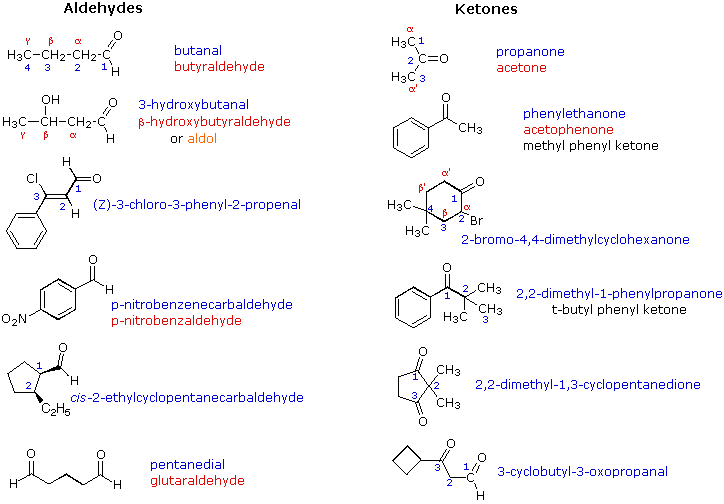 organicchemistry4fsc.blogspot.comketones chemistry aldehydes groups functional chain carbonyl examples group organic alcohols name two simple hydroxyl oh names reactivity propanone shown
organicchemistry4fsc.blogspot.comketones chemistry aldehydes groups functional chain carbonyl examples group organic alcohols name two simple hydroxyl oh names reactivity propanone shown
20.3: aldehydes, ketones, carboxylic acids, and esters. Aldehydes, ketones and carboxylic acids. Pin on organic chemistry